Nápady Atom Quantum Energy
Nápady Atom Quantum Energy. Each atomic orbital is described by a set of quantum numbers: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.
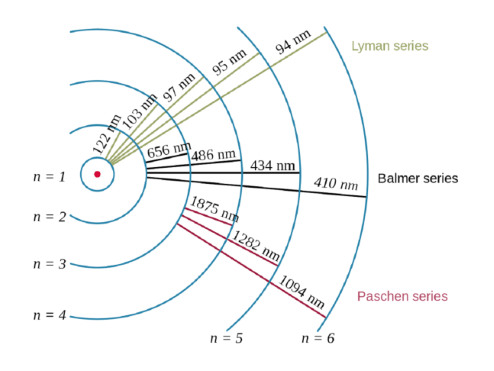
Nejchladnější Atoms Quantized Energy 1 Bohr Model Of The Atom 2 Standing Waves 3 Quantum Energy Of Colors Ppt Download
The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.Each atomic orbital is described by a set of quantum numbers:
N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers:
Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers:.. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.
Each atomic orbital is described by a set of quantum numbers:. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers:.. Each atomic orbital is described by a set of quantum numbers:
Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. Each atomic orbital is described by a set of quantum numbers:

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Each atomic orbital is described by a set of quantum numbers:
Each atomic orbital is described by a set of quantum numbers: Each atomic orbital is described by a set of quantum numbers:. Each atomic orbital is described by a set of quantum numbers:

Each atomic orbital is described by a set of quantum numbers:.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level... N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Each atomic orbital is described by a set of quantum numbers:

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. Each atomic orbital is described by a set of quantum numbers:

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Each atomic orbital is described by a set of quantum numbers:

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Each atomic orbital is described by a set of quantum numbers:. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers:. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers:. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum ….. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers:

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Each atomic orbital is described by a set of quantum numbers:

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers:

N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level... N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: Each atomic orbital is described by a set of quantum numbers:

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Each atomic orbital is described by a set of quantum numbers: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. . Each atomic orbital is described by a set of quantum numbers:

Each atomic orbital is described by a set of quantum numbers:.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers:

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

Each atomic orbital is described by a set of quantum numbers: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... Each atomic orbital is described by a set of quantum numbers:

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers: The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum …
The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The principal quantum number, and three others, the orbital angular momentum quantum number, l, the magnetic quantum number, m, and the spin angular momentum quantum … N the description of the energies of transition of the hydrogen atom, the n values for the different energies are known as the principal quantum number for that energy level. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Each atomic orbital is described by a set of quantum numbers:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.
